Emotional
Does Topical Ketamine Work? – Cannadelics
Published
1 year agoon
By
admin
Ketamine can be taken intravenously, via IM, by mouth, by nose (snorted), and even as a topical treatment. How well does this last option work? Read on.
Ketamine basics
Ketamine isn’t a natural compound, but rather, a lab-made drug that was created in 1962 by pharmaceutical company Parke-Davis. In 1970 it received authorization by the US’s FDA as a Schedule III anesthetic for both humans and animals. Ketamine is different from other hallucinogens we speak about, because its actually legal for medical use. We are not talking about a Schedule I drug.
Besides its capability as an anesthetic, which is used for surgery, the drug also shows ability for cataleptic and analgesic effects. Though it causes sedation in users, its not actually a hypnotic, meaning, it doesn’t put a person to sleep. It also doesn’t cause a lowering of vital signs like blood pressure or respiration rate at general anesthetic levels, meaning its quite safe in terms of overdosing.
When ketamine was first tested back in the 1960’s, it was used on a prisoner population. At that time it was established that ketamine had both pain-relieving properties, and some kind of ability to affect mood. In terms of the first point, it went on to be used on the fields of Vietnam. In terms of the second, its been the center of a therapeutic market for several years as an alternative to standard antidepressants for mental health issues. Yet, somehow, there is no further official clearance given for these uses.
Even so, ketamine is used for both. Its legal status opens up a loophole, in that doctor’s can prescribe it for off label uses, if they think the medicine is applicable for the patient. This has led to a large gray market ketamine industry that functions outside the regular medical system. As of yet, there has been no catastrophe, or even near-catastrophe, despite smear-campaigns and warnings by the government.
Topical ketamine
Ketamine is known mainly as a drug you snort on the street. It’s powder, like cocaine, and you can make lines in the same way, or take it as a bump off a fingernail. For most people that do ketamine, this is the way they take it in. When used in a medical setting, however, its generally IV, since its usually used for anesthesia. It can also be injected into a muscle (IM), or taken as a pill; which has become big for at-home treatments.
The delivery method which is spoken about the least, is topical ketamine, but this exists as well. This simply means that ketamine is made into a cream, and put on the skin. While we hear about other methods of ketamine use, we rarely hear about this one. However, it exists, and there is some limited research into it, and current research underway.
In this study from 2015, entitled Analgesic effects of topical ketamine, study authors make a few points, like that ketamine is a good option for neuropathic pain and complex regional pain syndrome, since it doesn’t have serious side effects. They point out that clinically significant side effects are incredibly rare, even at high doses.
It seems there are no large scale studies for this, even though ketamine shows as a good (no, great) alternative to opioids, and the addiction and death issues therein. However, in this study of five patients with sympathetically maintained pain, all showed 65-100% reduction in pain scores, compared to pre-treatment levels. This study of 300 emergency room pain patients from 2021 showed that ketamine reduced pain symptoms; although it performed about the same as lidocaine.
In this 2021 review by Frontiers in Science, called Ketamine Use for Cancer and Chronic Pain Management it states, “Ketamine has repetitively been shown to reduce pain scores and subjective measures of pain. While the use of topical ketamine alone does not have strong supporting evidence, its use in multi-agent creams has been effective in some pain conditions.”
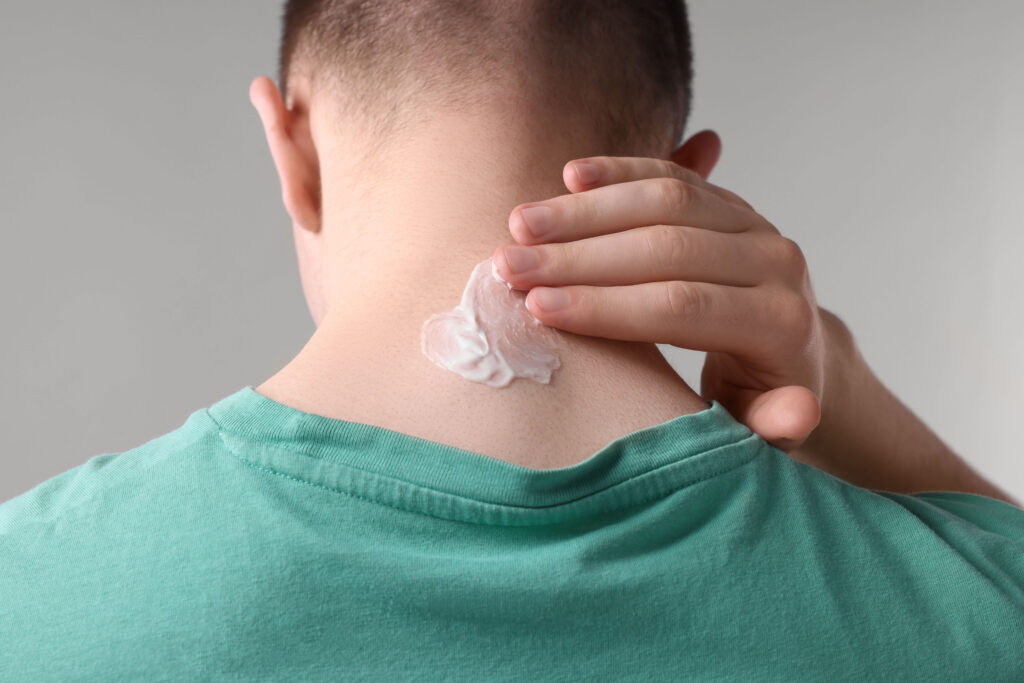
Topical ketamine for PTSD
New testing is underway to see if a topical ketamine product can help with psychological issues, like PTSD. In 2023, it was reported in Clinical Trials Arena, that the company Psycheceutical announced the start of Phase I trials into a new topical ketamine drug meant to treat PTSD, with Phase II trials already on the horizon.
The product, called NeuroDirect, is made to target nerve endings in the neck. This is meant it get around the hallucinogenic effects associated with the drug. How well it works to do so, is a big part of the current trials. They’re meant to assess how safe the drug is, how well its tolerated, and the general pharmacokinetics; all after a single dose. Only 24 patients are included in these trials, however, all are healthy patients. No PTSD in this population
This is the first set of trials that look into whether a topical version of ketamine is safe for a consumer population. Prior to these trials, investigators’ pre-clinical observational results, showed that approximately 80% of participants gained a benefit for their PTSD, from the topical ketamine.
So far, the Phase I participants were all successfully dosed, and the dosing was tolerated fine. Should subsequent trials also go well, this new medication would make ketamine administration easier and safer, since it wouldn’t involve directly entering anything into the bloodstream. Oral use is also an option, but is hard to predict outcomes with, as different patients absorb it quite differently.
Psycheceutical’s CEO Chad Harman, had this to say about the undertaking: “With our goal of a non-systemic treatment for PTSD using legal ketamine, we are attempting to pave the way for our future drug candidates to treat other mental health disorders.”
In terms of the intention for Phase II trials, these would revolve around using the NeuroDirect on PTSD patients. As per an announcement in February of last year, the company is looking to enlist 115 study participants with PTSD, to assess how effective the drug is in reducing PTSD symptoms.

Main benefits of topical ketamine
There are several benefits to using a topical version of ketamine over injected versions or oral versions. For one, its easy to use, and requires nothing more than applying to the skin. It’s just as easy to take a pill, of course, but pills come with the detraction of not knowing how a specific person’s body will break it down and respond.
The topical ketamine is put directly on the back of the neck, because this puts it close to the brainstem. Here, a drug can enter the body without going through the blood-brain barrier. This direct approach is meant to bring down unwanted side effects related to nausea, dizziness, or hallucinations. It does not have to be processed through the digestive tract, like a pill does.
Since the drug uses such a direct application, it can be with a low dose, which brings down any risk of addiction. Realistically, ketamine is rarely spoken about as an addictive drug, except when it comes to companies or governments telling you what product to buy. However, if this idea satiates those entities, all the better.
The simple application method means that patients don’t have to be in a hospital setting, and essentially gives another option for at-home ketamine use. It’s hoped this will bring down costs both for individuals and insurance companies; and allow more people access to these treatments.
Conclusion
For whatever reason we don’t see many topical ketamine products; it seems this is about to change. In the next few years, I expect this product will come to market, as well as plenty of others, as ketamine only grows in popularity. Both for pain issues (particularly as a way to avoid opioids) and the treatment of mental health disorders.
Hello all! We welcome you to Cannadelics.com, an independent rag in the wellness space, here with the best stories of today. Come by daily to be a part of everything; and subscribe to the Cannadelics Weekly Newsletter, for all the best news and product promos.
Related
You may like
channa
Kanna – What It Is, And How It Can Help You
Published
1 year agoon
January 21, 2024By
admin
The world of natural medicine is full of plants to promote wellness; here’s a little on kanna, and what it can do for you.
What is kanna?
At first glance, it might look like this is a shortened version of ‘cannabis’, spelled with a ‘k’ rather than a ‘c’. If you say it out loud it also sounds like you’re right about to say ‘cannabis.’ In reality, the two plants have very little in common except a name that sounds a bit similar.
Kanna is technically named Mesembryanthemum tortuosum, or Sceletium tortuosum, which are both obviously quite a mouthful. It also goes by the nicknames channa, and kougoed; the latter of which translates to ‘something to chew.’ It’s a succulent plant that hails from South Africa, particularly the Cape Provinces, where it was used primarily by the San and Khoikhoi peoples. It’s in the Aizoaceae family of plants.
The plant has small full leaves (as it is a succulent), and yellow and white flowers. The flowers are more yellow in the center and white around the outsides; and the petals are long and thin, and resemble spears shooting out from the center.

The plant has been used in South Africa since pre-historic times, or at the very least, a super long time. It wasn’t written about formally until 1662, when Dutch navigator Jan van Riebeeck first mentioned something about its use. The plant is usually dried and then chewed; although it can be made into a tea, or a snuff to smoke. In modern times, it’s often seen as a capsule, powder, or tincture, as well.
Traditionally, the plant was/is used to deal with issues like stress, and depression. Native cultures use it to promote relaxation and general wellbeing. It was/is also used as a pain medication, and as a way to suppress the appetite. Furthermore, it’s been studied for its ability to help dogs and cats which are suffering from dementia, from barking or meowing excessively at night.
Is kanna psychoactive?
Oftentimes, a plant’s name is derived from a major (or important) component within it. Such is the case for Mesembryanthemum tortuosum, which contains the active compound mesembrine. Mesembrine is an alkaloid, with about .3% in the roots of the plant, and .86% in the leaves, stems, and flowers.
In research, this compound shows the ability to work as a serotonin reuptake inhibitor. This might sound familiar, as a major class of antidepressant drugs, is called ‘selective serotonin reuptake inhibitors;’ which indicates the plant might have some of the same benefits. Common SSRIs include the heavily prescribed Zoloft and Prozac. As kanna has been used traditionally to combat stress and depression, this connection makes sense. It also makes it a natural version of what pharmaceutical companies produce.
Beyond this, mesembrine has also shown some ability as a weak inhibitor of the enzyme phosphodiesterase 4. Such drugs are associated with memory improvement, anti-inflammatory effects, increased wakefulness, and neuroprotective qualities. They’re thought to be possibly beneficial for a range of disorders, from depression, to Parkinson’s disease and Alzheimer’s disease, to multiple sclerosis, autism, strokes, and more.
All together, this indicates (along with recent research), that mesembrine, and the kanna plant as a whole, might be able to offer some solid benefits in terms of depression and anxiety management, as well as a treatment (whether alone or in conjunction with other compounds) for a range of other neurological impairments, pain issues, and inflammatory problems.

The plant contains another alkaloid called mesembrenone, which is thought to produce similar effects to mesembrine; and which is also thought to promote adaptogenic and antimicrobrial properties. Most plants that cause psychoactive effects (or really any medical effect), generally do so as a combination of compounds, not just one. In the world of cannabis, we specifically call this the entourage effect, but in general medicine its known as a synergistic effect.
What about pain?
Right now, pain is a particularly big topic in the US, as the desire to reduce it, led to what is one of the worst drug epidemics (essentially the worst) to befall civilization. Opioids certainly have a large recreational-only following, but they’re primarily pain medications. And it was their prescription for pain, that led to the mess we’re in. As such, pretty much anything that can help the pain issue, without causing the same problems of addiction, is greatly needed.
Another interesting compound in the plant is mesembrenol. This compound is associated with analgesic (pain-relieving) properties. Both this compound and mesembrine are thought to aid in pain reduction; and with none of the addictive side effects as synthetic opioid medications.
Traditionally, these pain-killing benefits were used mainly by native South Africans to treat headache pressure, abdominal pain, toothaches, for pain in the respiratory tract, and as a local anesthetic. In 2014 mesembrine’s analgesic properties were tested in rats, which were given up to 5000 mg/kg per day, with no adverse reactions. Investigators concluded that mesembrine “appears to have analgesic properties without abuse liabilities or ataxia.”
Human research into kanna safety
Before the rat study in 2014, a study came out in 2013, which investigated how safe and tolerable two different doses of kanna are for humans. The study, called A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial of Extract Sceletium tortuosum (Zembrin) in Healthy Adults used doses of 8mg and 25mg, which were given to participants once a day. The study lasted three months, and used all healthy adults. 37 people participated in the study.
The investigation was a randomized, double-blind, parallel-group, placebo-controlled, single center study. Let’s break this down. First off, there was a placebo group, which means some participants were given the kanna, and some were given an inactive compound. The randomized part means participants were randomly picked for the kanna or placebo groupings; and the double-blind part means neither the researchers nor participants knew which group they were in.

In terms of the parallel group part, this refers to some in the kanna group getting a smaller dose, and some getting a bigger dose; and that individual participants were given the same amount throughout the study. The last part, single center, refers to the study being conducted in only one location. 12 participants received 8mg kanna daily, 12 received 25 mg kanna daily, and 13 received the placebo daily.
Researchers found both doses of kanna to be tolerable. The most complained about adverse response was headache, followed by abdominal pain, and infections in the upper respiratory tract. However, more complaints came from the placebo group than either kanna group; indicating the complaints had little-to-nothing to do with the kanna.
In terms of unsolicited positive benefits (written in the journals of some participants taking kanna), these indicate increased feelings of wellbeing, and an improved ability to manage stress and sleep. As the study didn’t technically look into the effects or benefits of the drug, these unsolicited journal responses are the most that the study can show on the therapeutic front. Otherwise, it was mainly to assess safety and tolerability.
In terms of other physiological aspects, the kanna groups showed no difference in ECG, body weight, or in their physical examinations, which were all taken in the beginning and the end of the study. There were also no changes in hematology or biochemistry metrics, indicating the drug did little to change the body physiologically. Overall, kanna showed to be a physically safe drug at doses up to 25mg a day, regardless of therapeutic ability.
Conclusion
Kanna seems to offer a multitude of benefits to humans, like help with stress and depression, assisting in pain management, anti-inflammation benefits, and as an anti-microbial. Perhaps in the future we’ll see more of it; and perhaps big pharma will ensure that never happens.
Luckily, for those in the US who want to use this plant to help with a mental or physical issue, kanna, and its compounds, are currently perfectly legal. It can already be found in smart shops around the country; and as with anything else, interested users, should go at it responsibly.
Welcome wellness fans! Thanks for dropping by Cannadelics.com, where we cover the most interesting stories in the general wellness space. Stop by regularly to stay up on stories; and check out our Cannadelics Weekly Newsletter, for an assortment of awesome product promotions, as well.
Related
Emotional
Survey: Psychiatrists More Optimistic About Hallucinogen Therapies
Published
1 year agoon
October 24, 2023By
admin
Hallucinogen therapies are gaining a lot of traction with consumers, as well as with psychiatrists; as per the results of a new study.
Study on psychiatrists and hallucinogen therapies – setup
A new study, entitled American Psychiatrists’ Opinions About Classic Hallucinogens and Their Potential Therapeutic Applications: A 7-Year Follow-Up Survey, is a follow-up study to see the changing opinions of psychiatrists toward newer hallucinogen treatments, like psychedelics. It was recently published in Psychedelic Medicine.
The original study on psychiatrists’ opinions toward psychedelic and hallucinogen therapies, was conducted by the same group of researchers, back in 2016. This was before the mass trend toward legalization and decriminalization of these substances; and was even before the bulk of legal cannabis industries opened. It suffices to say that a lot has changed since this time; and this follow-up was meant to see how the changes in the years in between, have affected how those who treat mental disorders, feel about using such therapies.
The study assesses the level of optimism of US psychiatrists, toward using these treatments. The second study looked at the years 2022-2023; the first, at 2016. The same methodology was used for both the first study, and its follow-up. A survey was emailed to 1,000 members of the American Psychiatric Association; who were picked randomly. This included 750 attending psychiatrists, and 250 resident-fellows. For the second survey, this collection took place at the end of 2022 into the beginning of 2023.

Current responses were measured against 2016 responses using a non-parametric trend test. Investigators also used a multivariate logistic regression model to predict agreement with the incorporation of such therapies into respondent practices.
What did the study find?
Not everyone who receives a survey in life, actually fills it out. In this case, 13.1% of those who received the survey, completed it. This made for 131 participants. The respondents for this follow-up were identified as having similar demographics to respondents in the 2016 original survey.
According to study results, 80.9% believed either moderately or strongly, that hallucinogenic treatments are a promising therapeutic measure for several different psychiatric disorders. 60.8% also thought these treatments show promise for those suffering from substance use disorders as well. The latter means an interest in using these drugs for things like alcohol and opioid addiction.
Even greater percentages wanted to see more research into these topics, signaling an interest in them, even if not to the point of fully approving them just yet. 93.9% strongly or moderately desired more research into their use for psychiatric disorders; while 88.6% wanted to see more research concerning research into their use for substance use issues. In terms of federal funding for clinical trials to better understand these topics; 84.7% supported this for psychiatric disorders, while 80.9% did for drug abuse problems.
These numbers show increased optimism for these therapies in comparison to the 2016 survey. They also show a decrease in fears over using these treatments for patients. Results showed that 50.4% showed a moderate to strong desire to include psychedelic and hallucinogenic-assisted therapies into their own practices.
Investigators concluded from the new survey, that “Our data reveal a striking positive shift in attitudes toward the therapeutic potential of hallucinogens among American psychiatrists since 2016, with a majority of responding psychiatrists planning to incorporate hallucinogen-assisted therapy into their practice if regulatory approval is granted.”

Big changes from 2016
When looking at a comparison like this, it’s important to know about the original study. After all, we want to know what this new comparison, is a comparison to. The original study, called A Survey of American Psychiatrists’ Attitudes Toward Classic Hallucinogens, collected data in 2016, but was published in 2018, in The Journal of Nervous and Mental Disease.
At the time of information collection, the idea of hallucinogenic therapies had been reintroduced to society; but was still way far behind our current acceptance level and understanding. There was considerably less recent research on these topics, at the time that the original survey was given out.
This survey was also sent to 1,000 randomly selected members of the American Psychiatric Association; with the same breakdown of resident-fellows (250) and attending psychiatrists (750). One big difference is that the response rate was much higher for the original; with 32.4% filling out the survey. This means 324 respondents, which is way more than the 131 who answered the follow-up.
According to the researchers about this survey, “Respondents tended to perceive hallucinogens as potentially hazardous and appropriately illegal for recreational purposes.” Many fewer saw hallucinogenic therapies as a positive form of psychiatric treatment; amounting to what researchers called a “large minority,” who did express some optimism.
This investigation found that resident-fellows, and male respondents, were more likely to have greater optimism toward using these treatments, and less overall fear; than female respondents and attending psychiatrists. The results also indicate that younger respondents were more positive about these treatments; which might indicate a generational divide when it comes to the acceptance of these therapies. Younger respondents may have experienced less negative jargon surrounding these drugs and their therapeutic uses, than older respondents.
Are hallucinogenic and psychedelic therapies the same?
If you’ll notice, both study titles involve the word ‘hallucinogens,’ not ‘psychedelics.’ However, in a lot of media reporting, its only the term ‘psychedelics,’ that’s used. What’s the difference? The truth is, there is overlap in the meaning of these words; but they do not mean the same thing.

Basically, all psychedelics are hallucinogens, but not all hallucinogens are psychedelics. This automatically means that all psychedelic therapies, are included when looking at hallucinogen treatments; which encompass psilocybin (from magic mushrooms), DMT, mescaline, and LSD. Even MDMA, which is often included in the ‘psychedelics’ category, is technically classified as a ‘psycho-stimulant.’ However, its still relevant to this study, since it’s a hallucinogen. ‘Psychedelics’ is often used as a catch-all phrase these days, to represent hallucinogenic drugs in general.
Psychedelics are one of three main classes of hallucinogens; together with deliriants (like datura or Benadryl), and dissociative (which include ketamine, PCP, and DXM.) They are generally separated by their mode of action in the brain, and the neurotransmitters they affect most. Psychedelics cause the biggest force at serotonin receptors, deliriants do at acetylcholine receptors, and dissociative drugs at NMDA receptors. These are simplifications, as the drugs can affect different parts of the brain; but it gives an idea of the classification breakdown.
Other hallucinogens exist, which are not in these categories. Like MDMA, which for the most part works similarly to psychedelics, but with a greater stimulant effect; salvia, which affects opioid and dopamine receptors; and amanita mushrooms, which have a strong effect at GABA receptors. These are up-and-coming substances, but the plant world offers tons of other compounds; some of the names of which, are barely recognizable to the public. And some, like blue lotus, and ibogaine, which are starting to enter the conversation, too.
Hallucinogen drugs are defined as “psychoactive substances that produce powerful alterations in perception, mood, and various cognitive processes.” A hallucination, is an experience through the senses, of something that is not real, or altered from reality. This means seeing, hearing, smelling, tasting, or feeling something, in a way that is not actually happening. A hallucinogen is any drug from any class, which produces these effects.
Conclusion
Plenty of psychiatrists are still not up for using hallucinogen therapies, like psychedelics, to treat psychiatric issues. And plenty are interested, but want more information. What is most interesting about the current follow-up, is the sheer change in attitude within less than a decade of time. If the increase was so great from 2016 to 2023; it seems quite possible that by 2030, these therapies will be a norm in the psychiatric treatment world.
Welcome one and all; thanks for being a part of Cannadelics.com; where we bring you cutting-edge reporting on the world of drugs at large – with a particular focus on cannabis and hallucinogens. Head our way daily to catch the updates; and sign up to our Cannadelics Weekly Newsletter; so you’re always aware of the biggest stories.
Related
Emotional
FDA Warning to Crack Down on Ketamine Industry
Published
1 year agoon
October 15, 2023By
admin
The US government does not like industries it cannot control and regulate. Case in point, ketamine; and a new warning by the feds to crack down on the industry.
FDA warning letter about ketamine
Sometimes the US government can’t do much but hope people listen to a directive. Such is the case now concerning the ketamine industry, and a recent warning letter issued by the FDA. The letter came out on October 10th, 2023. Unlike some letters that I report on that have to do with warning producers about legal consequences, this letter is a bit different; because the circumstances are different. This letter is geared toward consumers and practitioners.
Its not a cease and desist letter, like the ones that get sent to cannabinoid companies that sell products like delta-8. Instead, the letter acts more as a warning for the health and well being for those interested in ketamine. The letter highlights the following points:
- It reminds that ketamine is not an FDA approved medicine for psychiatric disorders. It explains that the FDA has not decided that compounded ketamine – currently used for the treatment of a range of mental issues from depression to PTSD to OCD, is safe for these uses.

- It’s second point is that compounded drugs in general are not FDA approved. A compounded drug is one that’s prepared specifically for a client. These medications are tailored to the particular needs of an individual by a medical professional; something that is offered at hospitals or pharmacies. They are not standardized products. It goes on to stipulate that compounded medications can be okay, when used under the care of a healthcare provider.
- It’s third point is that using such compounded ketamine medication should always be under the supervision of a doctor; to ensure sedation, dissociation, and vital signs like blood pressure, don’t get out of whack. It warns of serious adverse events with these specific things.
- Then it goes on to push its own ketamine (esketamine) over other ketamine products. In fact, it says that while other products can be cause for concern, that if you use the FDA approved medication in the right dosing, that the benefits outweigh the risks. The FDA is seemingly saying that things like “misuse, psychiatric events, increases in blood pressure, respiratory depression (slowed breathing), and lower urinary tract and bladder symptoms” are associated with non-FDA approved products.
- It’s last point is that it (the FDA), is not aware of any benefit of unapproved ketamine products, that make them better than FDA approved medications. I assume it means either its version of ketamine, or standard anti-depressants.
Does this make sense?
Of course not. An FDA approval means the government decided something is safe, with the implication that without this approval, something is not safe. But this is massively antithetical considering the ketamine industry is not associated with death or injury rates; while FDA-approved medications like fentanyl (and other synthetic opioids), are.
Beyond the FDA offering up such synthetic opioids, it also offers a host of benzodiazepines, and tons of other pharmaceuticals; like Tylenol, blood pressure medication, and antidepressants; which are fingered for their danger. Tons of FDA products are constantly recalled after approval, because of how not safe they are. Let’s be honest, the FDA still holds cannabis as Schedule I; which really speaks to the lack of logic and medical backing, of any of this.
The warning letter speaks of the ketamine industry as if its illicit; but its most certainly not. In fact, it’s a medical industry that requires prescription, and is overseen by medical professionals. This makes the bulk of the letter pretty useless; since its warning against non-medical use, in what is a medical industry. It warns of needing doctor supervision; but that’s a standard aspect of the industry, creating another moot point. Perhaps the FDA assumes you don’t know it’s a medical industry.

Beyond that, ketamine simply isn’t associated with all the issues the FDA warns about; or at least, not in any kind of detrimental way. It’s not a drug of high abuse; it has no death toll attached; and the reason it originally came into view, is because of its ability for use as an anesthesia, without causing major impact to functions like blood pressure. And the FDA knows all this, because there is a wealth of information on ketamine; starting with prisoner studies from the 1960’s, where it was first discovered to affect mood, and to anesthetize safely.
It’s last point, that ketamine hasn’t shown to be more effective than approved medications, is funny at best. For one thing, the letter pushes the government approved version of ketamine; which means it does see a benefit in it. And if it really wants to compare ketamine to antidepressants, well, ketamine routinely shows direct positive effects, whereas after all these years, no one can say anything definitive about the usefulness of antidepressants. In fact, there are plenty of concerns that they can cause mood disturbances.
The ketamine loophole
Why does this warning letter about ketamine really exist? Because the bulk of the ketamine industry, is not government regulated. It is medical, but it exists outside of the standard medical system. Ketamine stands as one of the few drugs capable of doing this; and has created a loophole for medical use beyond what its approved for.
The reason is that ketamine isn’t a Schedule I drug. It’s a Schedule III drug which is clear for medical use as an anesthesia. The thing is, the FDA allows off-label prescribing of any authorized drug. This means “the use of pharmaceutical drugs for an unapproved indication or in an unapproved age group, dosage, or route of administration. Both prescription drugs and over-the-counter drugs (OTCs) can be used in off-label ways, although most studies of off-label use focus on prescription drugs.”
The way it works is that “Once the FDA approves a drug, healthcare providers generally may prescribe the drug for an unapproved use when they judge that it is medically appropriate for their patient.” As it can only be prescribed by a doctor, the current FDA letter is essentially trying to tell doctors, what they should and should not prescribe. In fact, its attempting to tell medical professionals, what is safe and what is not.
Ketamine clinics function as legal medical clinics; that’s why this is not a cease and desist letter. All warnings within are fear warnings over safety, with no evidence offered to back anything up; and no warning of punishment. It does not include anything about legal backlash. It’s more like an attempt to put pressure on doctors to stop prescribing, and to get consumers afraid of going to ketamine clinics.

This is likely because the US government isn’t regulating the industry, which also means it can’t attach extra taxes, or regulatory fees. Much like with the vape industry, and the weed industry; no government wants products out there that it can’t collect on. While standard taxes are paid by ketamine providers, there is nothing supplemental attached for the use of ketamine, like sin taxes; and no specific costly regulatory fees to collect. And let’s be honest, considering the government approved version requires the user be on standard antidepressant to access it; few people are likely to care about it.
Is a letter like this likely to change anything? Not really. It only exists as a means of reigning in something that is already popular; and for which enough people have found benefit, to keep it going. These letters have done nothing to stop vape manufactures, cannabinoid manufacturers, or providers of these products; and that’s with cease and desist letters. A simple request with no threat? It’s like it didn’t happen at all.
Conclusion
Ketamine therapy might not be right for everyone; but there’s plenty of evidence of its usefulness; and it certainly isn’t associated with death or destruction, with addiction, or with bad behavior. The biggest negative of the ketamine industry is that its high pricing often rules out clientele; something which is most certainly an issue in the burgeoning mushroom industry as well.
Welcome to the publication. You’ve arrived at Cannadelics.com; an independent news source in the general drug space, here to bring you the lowdown on the most interesting stories going on. Come around daily to catch your updates; and sign up for our Cannadelics Weekly Newsletter; to access prime product promotions, along with the news.
Related

This Wine Issue Is Becoming More Common

Get more for less this 420 at PurLife

Can You Order Cannabis through DoorDash?

Cannabis Enthusiast-Friendy Retreats – GanjaVacations Jamaica

The road ahead for cannabis lending in 2025

Cannara Biotech Announces Appointment of Justin Cohen to Board of Directors
Can Marijuana Help Cholesterol – The Fresh Toast

Safe Cannabis Consumption During Wildfire Season

Food Asphyxiation Is Way More Dangerous Than Cannabis

Outdoor Marijuana Grows Are Better All The Way Around

Distressed Cannabis Business Takeaways – Canna Law Blog™

United States: Alex Malyshev And Melinda Fellner Discuss The Intersection Of Tax And Cannabis In New Video Series – Part VI: Licensing (Video)

What you Need to Know
Drug Testing for Marijuana – The Joint Blog

NCIA Write About Their Equity Scholarship Program

It has been a wild news week – here’s how CBD and weed can help you relax

Cannabis, alcohol firm SNDL loses CA$372.4 million in 2022

A new April 20 cannabis contest includes a $40,000 purse

Your Go-To Source for Cannabis Logos and Designs

UArizona launches online cannabis compliance online course
Trending
-

 Cannabis News2 years ago
Cannabis News2 years agoDistressed Cannabis Business Takeaways – Canna Law Blog™
-
 One-Hit Wonders2 years ago
One-Hit Wonders2 years agoUnited States: Alex Malyshev And Melinda Fellner Discuss The Intersection Of Tax And Cannabis In New Video Series – Part VI: Licensing (Video)
-

 Cannabis 1012 years ago
Cannabis 1012 years agoWhat you Need to Know
-

 drug testing1 year ago
drug testing1 year agoDrug Testing for Marijuana – The Joint Blog
-

 Education2 years ago
Education2 years agoNCIA Write About Their Equity Scholarship Program
-

 Cannabis2 years ago
Cannabis2 years agoIt has been a wild news week – here’s how CBD and weed can help you relax
-

 Marijuana Business Daily2 years ago
Marijuana Business Daily2 years agoCannabis, alcohol firm SNDL loses CA$372.4 million in 2022
-

 California2 years ago
California2 years agoA new April 20 cannabis contest includes a $40,000 purse



