Cannabis News
Why Weed is the Best Thing That’s Happened to Endometrosis Patients in the Last 50 Years
Published
3 months agoon
By
admin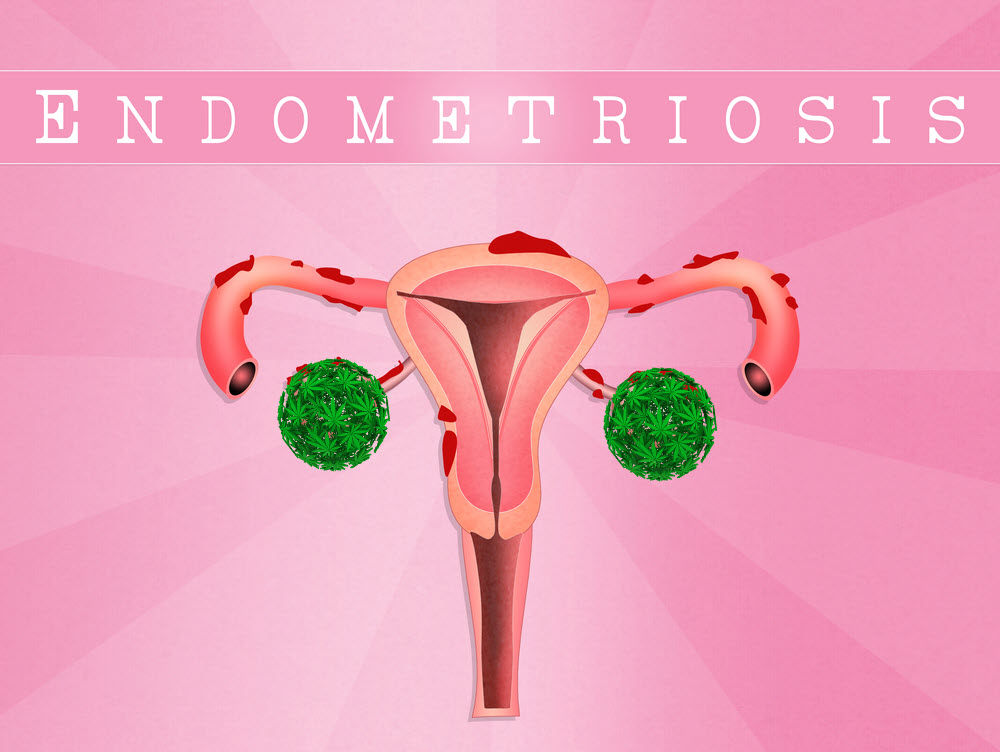

Endometriosis is one of the most pain conditions affecting women worldwide.
When endometriosis occurs, the endometrial tissue which normally grows inside the uterus, grows outside of it. As a result, it can attach itself to other reproductive organs in the area. In extreme cases, endometriosis can reach far beyond the reproductive area and cause serious pain and damage. The symptoms of endometriosis include sharp period pains, fatigue, pain during sex, infertility, heavy periods, and much more.
According to the World Health Organization, around 10% of women who are in reproductive age, have endometriosis. Unfortunately, there is no known cure, though several protocols can be integrated into a patient’s lifestyle to mitigate or reduce symptoms. Some common medications used for reducing the symptoms of endometriosis include birth control pills, hormonal injections, patches, and intra-uterine devices (IUD), to name a few. However, these may affect a woman’s chances of getting pregnant, or make infertility even worse since endometriosis can also affect fertility.
Thankfully, more women are finding success with lifestyle changes and integrating natural treatments to treat endometriosis. Along with regular exercise, weight loss among overweight women, and healthy habits, cannabis has been shown to be helpful treating this dreaded disease.
What Studies Are Saying
There are more studies than ever, too, proving the efficacy and benefits of cannabis for women suffering from endometriosis. In a recent study out of Germany, researchers analyzed survey data from more than 900 endometriosis patients to determine how, if any, the symptoms are impacted by cannabis.
A significant percentage of the 900 respondents acknowledged using marijuana, and most of them said that it was beneficial in helping with symptoms. “Seventeen percent of the respondents used cannabis flower strains or cannabis-related products as a self-management method,” said the researchers. “Cannabis was rated as the most effective self-management strategy to reduce symptom intensity (self-rated efficacy 7.6 out of 10). Additionally, ~90 percent of the participants were able to decrease the pain medication intake,” explained the researchers. They also noted that the participants observed the most significant benefits from cannabis in their sleep, menstrual pain, and non-cyclic pain in that order. Because of this, it’s become clear that cannabis can greatly help improve quality of life in these areas, which are otherwise affected by symptoms of endometriosis.
It’s important to note that the investigators also acknowledge that existing studies on cannabis for endometriosis is limited, yet there is an increasing quantity of studies pointing to a growing trend of women self-medicating with cannabis for this condition, and seeing success!
“The use of cannabis has a significant impact on the overall well-being and quality of life of women with endometriosis,” they concluded. “The study indicates that there is a significant interest and demand for additional therapeutic options, and cannabis can potentially become an important part of a multimodal therapy approach for treating endometriosis,” said the study.
Another study from Australia, whose results were published earlier this year, found similar results. The data, which was published in the journal, Obstetrics and Gynecology, were taken from 192 women who were polled by Australian researchers regarding their history of marijuana consumption and endometriosis symptoms.
According to the findings, 63% of the poll respondents were given doctor’s authorization letters for using cannabis-based medications. In Australia, physicians are legally allowed to recommend cannabis use for patients who have had no luck treating specific conditions using conventional prescription drugs. In the study, most women were using THC-dominant marijuana products and they reported improvements when it came to sleep, pain, nausea, and anxiety.
“This study found that THC-predominant CBMPs [cannabis-based medicinal products] are commonly prescribed to Australians with endometriosis,” said the authors. “Given major issues with symptom management and the self-reported reductions in pain and other symptoms, improving access to medicinal cannabis for this population is important and timely,” they said.
Why Cannabis Works So Well For Endometriosis
These studies and tons of anecdotal evidence available on the internet show that cannabis is indeed safe and effective for treating endometriosis. After all, cannabis interacts with the endocannabinoid system (ECS), which plays an important role in helping us regulate pain, inflammation, and our immune response – all of which are impacted by endometriosis.
While there are many symptoms that cannabis can help with, one of its greatest successes has been in reducing pain. Women have found success using both high-CBD and high-THC products to reduce the severe pain that accompanies endometriosis. Likewise, the pain, discomfort, and cramping can make it difficult to get some decent sleep; marijuana can help with that too!
In addition, marijuana can help women’s bodies balance out hormones, since the endocannabinoid system also plays a role in hormone regulation.
Furthermore, women can choose from a variety of products to help treat endometriosis. While smoking or vaping is always a popular choice, there ae also edibles, topical creams, oils, beverages, and tinctures.
Conclusion
When looking to treat endometriosis, remember that there is no miracle cure out there. Marijuana certainly helps, but it’s not a one-size fits-all approach. Please remember to consult with a medical professional, and keep in mind that you will always have the best results if you also address other lifestyle factors as mentioned earlier.
Maintaining a healthy weight, avoiding processed and sugary food, and regular exercise are all key factors to mitigating the symptoms of endometriosis. Add cannabis to the mix, and you can now look forward to regaining a better quality of life.
MARIJUANA STRAINS FOR ENDO PATIENTS, READ ON…
You may like
-


What Does the Future Look Like in an AI Dominated World?
-


Cannabis testing lab fraud can be curbed if industry is ‘part of the solution’
-


The Oregonian: Naked Oregon man wounded by exploding weed pen, lawsuit claims
-


The Best Thoughtful And Inexpensive Valentine’s Day Gifts
-


5 Ideas To Have A Fun Single’s Valentine’s Day
-


Snoop, Wiz, Lil Baby’s latest: Top celeb weed for 2025
Cannabis News
What Does the Future Look Like in an AI Dominated World?
Published
1 hour agoon
February 13, 2025By
admin

GANJA THEORIES: What does the future look like in 10-years time?
As I sit here, rolling up my evening joint and reflecting on the absolute circus that was 2024, I can’t help but marvel at how fucking weird everything has become. And folks, let me tell you – this is just the beginning. We’ve officially entered what I like to call “The Twilight Zone on steroids,” where reality seems to be shifting faster than you can say “artificial intelligence.”
Remember when the craziest thing we had to worry about was whether we’d get caught smoking behind the gym? Now we’re watching AI systems write poetry, create art, and probably plot their digital takeover while we sleep. Every morning I wake up to headlines that would have been rejected from Black Mirror episodes for being “too unrealistic” just a few years ago.
And let’s not forget about the testosterone-fueled war hawks, strutting around with their military-industrial complex boners, trying to convince us that World War III would be great for the economy. Meanwhile, conspiracy theories that seemed batshit insane last year are turning into tomorrow’s breaking news, and we’re all just supposed to act like this is normal.
So, I did what any reasonable cannabis enthusiast would do when confronted with the impending techno-apocalypse – I rolled up a fat one and let my mind wander into the future. What will our world look like in 2034? Will we all be working for AI overlords? Will cannabis finally be legal everywhere? Will we be fighting wars with robot soldiers, or will we have evolved beyond our primitive warfare instincts?
Fair warning: my predictions are probably going to be way off. But then again, who could have predicted where we are now? So spark up, settle in, and let’s take a trip into tomorrow. Trust me, it’s going to be one hell of a ride.
I recently stumbled upon a fascinating conversation between Zachary Levi and Glen Beck where they discussed the future of entertainment in an AI-dominated world. Levi, who’s surprisingly switched on for a Hollywood type, painted a picture that got my synapses firing – imagine a Disney+ where you’re not just watching content, but creating it. Want to see Indiana Jones and Captain America team up to take down Darth Vader while Bambi watches from the sidelines? Just type it in, and boom – instant custom movie.
Now, pass that joint for a moment, because while Levi’s vision is compelling, I think he’s missing a crucial point about human nature. We’re fundamentally lazy creatures. Sure, right now you can jump onto Midjourney or ChatGPT and create some mind-blowing content with minimal effort. Hell, I’ve seen AI-generated art that would make Salvador Dalí do a double-take. But here’s the thing – most people don’t want to create. They want to consume.
Think about it. After a long day of work, how many people actually fire up their creativity engines? Most folks just want to sink into their couch, crack open a beer (or spark up), and let entertainment wash over them like a warm wave of mindless comfort. They work their 9-to-5 to fund their 5-to-9 consumption habits. It’s the circle of modern life.
But here’s where things get really interesting – and by interesting, I mean potentially terrifying. What happens when AI starts automating away those 9-to-5 jobs? When robots are flipping burgers, driving trucks, and even writing code, what happens to all those consumers? We’re talking about a future where a significant chunk of people’s identity – their work – gets stripped away faster than papers at a Snoop Dogg concert.
See, work isn’t just about earning money. It’s about purpose, identity, and feeling like you contribute something to society. When I’m high, I often ponder this existential dilemma: if our jobs are what we do to afford the things we consume, what happens when we can’t get jobs anymore? The entertainment industry might be the canary in the coal mine, but it’s just the beginning. We’re staring down the barrel of a much bigger societal shift, and I’m not sure we’re ready for it.
Maybe it’s time we all took a deep breath (and a deep hit) and started seriously thinking about what it means to be human in a world where machines can do everything better than us. Because let’s face it – that world is coming faster than a pizza delivery during a serious case of the munchies.
Here’s a sobering thought that hits different after your third bong rip: there’s a monster lurking in our collective closet, and most people don’t even know it exists. I’m talking about the impending reality of mass unemployment due to automation. Not the gradual, manageable kind – I mean the “holy shit, what happened to all the jobs?” kind that’s barreling toward us like a runaway freight train.
Look, I’m not trying to harsh your mellow, but we need to talk about what happens when a significant portion of society suddenly finds themselves with nothing to do. Because let me tell you something – humans without purpose are like dried-out cannabis plants; they wither, they crack, and eventually, they become fuel for fire.
Speaking of fire, history has taught us what happens when large groups of purposeless people get together. All it takes is one charismatic asshole with a funky mustache or a weird haircut to start pointing fingers at “the other.” But this time around, “the other” won’t be some marginalized group – it’ll be the mega-corporations with their armies of AI robots, trained on data they harvested from us like we were their personal information farms. How’s that for irony? We basically taught our future overlords everything they know.
Now, I know what you’re thinking: “Damn, Reggie, that’s some dark shit.” And you’re right. This is definitely the kind of future you’d imagine after hitting some particularly paranoia-inducing sativa. But here’s the thing – we don’t have to slide face-first into this dystopian nightmare.
Maybe we pump the brakes a bit on this whole “automation revolution.” What if we implemented some kind of “human inclusivity” requirements? Think of it as affirmative action for the entire human race. “Sorry, RoboCorp, you need at least 30% meat-based employees to operate legally.” It sounds ridiculous, but so did the idea of carrying a supercomputer in your pocket just a few decades ago.
Or perhaps our roles evolve upward. Instead of packing boxes, we become logistics coordinators. Instead of flipping burgers, we become experience designers. We could shift into more complex, uniquely human roles that AI can’t easily replicate. At least not until they figure out how to simulate consciousness, but that’s a whole other joint we’ll have to smoke.
But here’s the kicker – who’s going to be making these decisions? Looking at our current crop of world leaders is about as reassuring as finding mold in your stash. These are the same people who can’t figure out if a plant should be legal or not, and we’re trusting them to navigate the biggest technological shift in human history?
I don’t know about you, but I’m thinking we’re going to need a lot more weed to get through this transition period. And maybe, just maybe, that’s not such a bad thing. After all, cannabis has a way of helping us see possibilities we might have missed otherwise. And right now, we need all the possibilities we can get.
If there’s one silver lining to this whole AI revolution – besides needing more cannabis to process it all – it’s that we’re entering an unprecedented age of individual empowerment. Picture this: you wake up with a vision, spark up your morning joint, and by sunset, you’ve created a short film complete with custom graphics, original music, and Morgan Freeman narrating (well, something that sounds eerily like him). That’s not science fiction anymore, folks. That’s Tuesday.
Sam Altman, the tech wizard behind OpenAI, predicted the first AI-enabled solopreneur billionaire. And you know what? He’s probably right. We’re watching creativity become a superpower right before our eyes. While everyone else is doom-scrolling through their social media feeds, the real players are out there mixing AI with human ingenuity like master alchemists, turning digital lead into gold.
Speaking of gold, originality is about to become the most precious commodity in a world where AI can churn out content faster than a hydroponic grow operation. When anyone can generate anything at any time, the truly unique, the genuinely human, will shine like a diamond in a pile of cubic zirconia. Entertainment won’t just evolve – it’ll mutate into forms we can’t even imagine yet. Music will break free from traditional structures. Art will explode into new dimensions.
And don’t get me started on science. We’re talking about discoveries dropping faster than new strain names at a cannabis cup. Every week bringing something that would have blown minds just a few years ago. It’s like humanity just upgraded from a bicycle to a spaceship, and we’re still figuring out which buttons do what.
Look, I can’t do anything about the power-hungry goblins running the show from their corporate towers. But what I can do – what we all can do – is dive deep into our own creativity and ride this wave of technological empowerment like cosmic surfers. Sure, the robots might take over the assembly lines, the customer service desks, and maybe even the corner offices. But they can’t replicate the spark of human inspiration (at least not yet).
The smart play here is adaptation. Take what you know, mix it with these new AI tools, and create something the machines couldn’t dream up on their own. Because let’s face it – a lot more jobs are going to disappear than most people realize. That’s not pessimism; that’s just reading the tea leaves (or in my case, the cannabis leaves).
But here’s my philosophy: instead of fighting the inevitable, I’m embracing it. I’ve stopped trying to control the uncontrollable and started focusing on creation, expression, and innovation. With every new AI tool that drops, I’m like a kid in a candy store, mixing and matching capabilities to bring my ideas to life.
We’re standing at the threshold of something massive here, folks. Humanity is about to level up in ways we can barely comprehend. The next decade will show us just how far this rabbit hole goes.
Of course, there’s always the possibility we’ll freak out and nuke ourselves back to the Stone Age. But hey, that’s just one possible timeline, right?
Your move, humanity. Choose wisely. And maybe keep some extra stash on hand – something tells me we’re going to need it.
REALISTIC LEGALIZATION FOR CANNABIS IN AMERICA, READ ON…
WHY IS 2033 REASONABLE FOR CANNABIS LEGALIZATION IN AMERICA?
Cannabis News
Hockey Players Ditching Post Game Beers for Cannabis Edibles and Playstations
Published
1 day agoon
February 12, 2025By
admin

The landscape of professional sports is constantly evolving, and the National Hockey League (NHL) is no exception. In recent years, a noticeable cultural shift has taken place within the league, as players increasingly turn away from traditional post-game celebrations centered around alcohol. Instead, they are embracing alternative forms of relaxation and entertainment, such as edible cannabis and video gaming. This trend reflects broader societal changes regarding health, wellness, and leisure activities, particularly among younger generations.
In this article, we will explore the factors contributing to this transformation in the NHL, examine its implications for players and the league, and consider what this means for the future of professional hockey.
A Changing Culture in Professional Sports
The Traditional Post-Game Scene
For decades, professional athletes have celebrated victories and coped with losses in locker rooms and bars with alcohol. The camaraderie built during these moments has been an integral part of team culture. However, with increasing awareness of the negative effects of excessive drinking—both on health and performance—many players are reconsidering their choices.
Rise of Health Consciousness
The shift away from alcohol consumption can be attributed to a growing emphasis on health and wellness in sports. Athletes today are more aware than ever of the importance of maintaining peak physical condition. With advancements in sports science and nutrition, players are focusing on optimizing their performance through better lifestyle choices.
In interviews, several NHL players have expressed their commitment to healthier living. For instance, Toronto Maple Leafs forward Mitch Marner has spoken about how social media has changed the way athletes engage with nightlife. “It’s different now,” he said. “You don’t want to be out there partying when you know everyone is watching.” This increased scrutiny has made players more cautious about their public personas.
The Emergence of Edible Cannabis
Legalization and Acceptance
One significant factor contributing to the rise of edible cannabis among NHL players is the changing legal landscape surrounding marijuana use. As more states in the U.S. and provinces in Canada legalize cannabis for recreational use, athletes feel more comfortable exploring its benefits without fear of repercussion.
The NHL has historically taken a hard stance against substance abuse; however, its policy on cannabis has evolved. The league no longer suspends players for positive tests related to marijuana use, recognizing its potential therapeutic benefits for pain management and recovery.
Therapeutic Benefits
Many players have turned to cannabis as a natural alternative to traditional painkillers or anti-inflammatory medications. Edible cannabis products offer a discreet way to consume THC without the stigma associated with smoking. Players report using these products to help with anxiety, sleep issues, and recovery from injuries.
For example, former NHL player Riley Cote has become an advocate for cannabis use in sports after his own experiences with pain management during his career. Cote emphasizes that cannabis can provide relief without the side effects associated with opioids or alcohol.
Video Gaming: A New Form of Socialization
Alongside the shift towards edible cannabis is the growing popularity of video games among NHL players. Esports have exploded in popularity over the last decade, providing a new avenue for social interaction and competition among athletes. Many players now spend their downtime playing video games together online rather than heading out for drinks.
This trend has been particularly pronounced during the COVID-19 pandemic when social distancing measures limited traditional forms of entertainment. Players found solace in gaming communities where they could connect with teammates and friends while staying safe at home.
Video gaming has also become a tool for building team chemistry. Many NHL teams now incorporate gaming sessions into their training regimens, allowing players to bond over shared interests outside of hockey. This informal setting fosters communication and teamwork skills that can translate into better performance on the ice.
Players like Nashville Predators’ Matt Duchene have spoken about how gaming helps them unwind while still maintaining a competitive edge. “It’s a great way to relax after games,” Duchene noted. “You can connect with guys without having to go out.”
The Impact on Player Relationships
As drinking culture declines within the NHL, player relationships are evolving as well. While alcohol often served as a social lubricant that brought teammates together after games, alternatives like gaming and cannabis are fostering new connections based on shared interests rather than shared drinks.
This shift may lead to deeper bonds among players who engage in activities that promote teamwork and camaraderie without the potential pitfalls associated with alcohol consumption. Players are finding new ways to support each other both on and off the ice.
The focus on healthier lifestyles also aligns with growing awareness around mental health issues in sports. Many athletes face immense pressure to perform at high levels, which can lead to anxiety and stress. By prioritizing mental well-being through alternative relaxation methods like gaming or cannabis use, players may find healthier coping mechanisms that contribute positively to their overall mental health.
League Response and Future Implications
The NHL’s evolving stance on cannabis reflects broader societal changes regarding its acceptance. As more research emerges about its benefits for athletes, it is likely that we will see further integration of cannabis into player wellness programs.
The league’s leadership has acknowledged these shifts by allowing teams to educate players about responsible cannabis use while also promoting healthy lifestyles overall. This proactive approach may help reduce stigma around cannabis use within professional sports.
As video gaming continues to gain traction among NHL players, teams may explore ways to incorporate technology into their training regimens further. Virtual reality (VR) training sessions or gamified drills could enhance player development while catering to their interests outside traditional practice methods.
Additionally, partnerships between NHL teams and gaming companies could lead to innovative fan engagement strategies that bridge the gap between hockey and esports communities.
Conclusion
The cultural shift within the NHL signifies a notable change from traditional post-game celebrations, as players increasingly favor healthier alternatives like edible cannabis and video gaming over alcohol, redefining how they celebrate victories and bond as teammates. This transformation reflects evolving attitudes towards health and underscores the growing emphasis on mental well-being in professional sports, with players prioritizing self-care through modern relaxation methods, thereby setting a precedent for future generations to follow. The NHL now stands at a pivotal moment where it can either hold onto outdated practices or embrace a new era that values player health and fosters deeper connections among teammates both on and off the ice; clearly, the trend is moving away from alcohol consumption towards integrating healthier options into the culture of professional hockey.
CANNABIS FOR SPORTS FANS, READ ON…
FIFA WANTS YOU TO SMOKE WEED AND NOT DRINK FOR THE WORLD CUP!
Cannabis News
How Does DOGE and the Federal Funding Freeze Impact the Cannabis Industry?
Published
2 days agoon
February 11, 2025By
admin

The cannabis industry, a burgeoning sector with significant economic potential, operates in a complex landscape shaped by evolving state regulations and persistent federal prohibition. Recent events involving a temporary federal funding freeze have highlighted the vulnerability of this industry, particularly for Indigenous communities actively involved in its development. While the immediate crisis was averted, the episode served as a stark reminder of the precariousness of relying on federal support and the urgent need for self-sustaining revenue models.
In late January 2025, the White House’s Office of Management and Budget (OMB) issued a memo calling for a temporary pause on payments for federal grants and other programs[1][4]. This decision, stemming from an executive order by then-President Trump, triggered widespread confusion and anxiety across various sectors, including housing, education, and nonprofits[1]. The Native American Rights Fund (NARF) quickly issued a statement emphasizing the disproportionate impact such a freeze would have on Tribal Nations. Fortunately, the Trump administration rescinded the memo just two days later, following a temporary pause on implementation by a federal judge. Another judge followed suit with a restraining order.
While the immediate threat was neutralized, the incident exposed the inherent risks associated with federal funding dependence, particularly for Indigenous communities striving to establish themselves in the cannabis industry. The Indigenous Cannabis Industry Association (ICIA), an organization uniting Native American tribes around the cannabis plant, released a statement addressing the situation and its implications.
Disproportionate Impact on Indigenous Communities
The ICIA’s founder, Rob Pero, a member of the Bad River Ojibwe and founder of Canndigenous, Wisconsin’s first independent Indigenous-owned hemp company, emphasized the disproportionate impact of a federal funding freeze on Indigenous communities. “The threat of a federal funding freeze has a disproportionate impact on Indigenous communities, exacerbating existing economic disparities,” Pero told Forbes. He explained that tribes with diversified economies, particularly those with established cannabis operations, are less vulnerable to the immediate effects of such a freeze because cannabis businesses operate without federal funding due to federal illegality.
However, the situation is drastically different for tribes heavily reliant on federal funding for essential services. Pero described these funds as “lifelines, not just budget items,” emphasizing that even a temporary freeze jeopardizes critical programs and highlights the precariousness of relying on federal support. The incident underscored the urgent need for Indigenous nations to develop self-sustaining revenue models to mitigate their vulnerability to external political decisions.
The Cannabis Industry as a Path to Economic Sovereignty
The cannabis and hemp industries offer unique advantages for tribes seeking economic independence. These advantages include regulatory sovereignty, geographic benefits, and cultural expertise. The ICIA encourages Indigenous communities to collaborate on cultivation, processing, distribution, and market access to create a self-reinforcing economic network benefiting all Indigenous nations, regardless of their stage of development in the cannabis or hemp industries. Pero envisions established operations mentoring and supporting those just beginning, ensuring that no tribe is left behind as these industries grow. This collaborative approach is not just about individual success but about collective economic sovereignty and reinforcing tribal self-determination for generations to come.
Federal Prohibition: An Ongoing Obstacle
The federal funding freeze saga highlights the broader challenges faced by the cannabis industry due to federal prohibition. Despite the growing number of states legalizing cannabis for medical and recreational use, the plant remains a Schedule I controlled substance under federal law. This creates numerous obstacles for cannabis businesses, including:
-
Limited Access to Financial Services: Many financial institutions are hesitant to provide services to cannabis businesses due to compliance concerns, forcing them to operate primarily in cash, which increases the risk of theft and makes it difficult to manage finances.
Potential Benefits of Federal Legalization
Federal legalization of cannabis could transform the U.S. cannabis market, unlocking its full economic potential and addressing many of the challenges currently faced by businesses.
Key benefits of federal legalization include:
-
Increased Access to Funding: Legalization would open the door for traditional financial institutions to provide loans and other financial services to cannabis businesses, increasing their access to capital.
-
Reduced Regulatory Burden: A national regulatory framework would replace the patchwork of state laws, reducing the regulatory burden on cannabis businesses and creating a more consistent and predictable business environment[2].
-
Greater Research Opportunities: Federal legalization would facilitate research into the potential benefits and risks of cannabis, leading to a better understanding of its effects on human health and well-being[3].
-
Increased Tax Revenue: Legalization would generate significant tax revenue for federal and state governments, which could be used to fund important public services[3]. In 2031 alone, the MORE Act revenue impact expectation is $1.4 billion[3].
The Path Forward: Towards Economic Sovereignty and Federal Reform
The temporary federal funding freeze served as a wake-up call, highlighting the vulnerability of the cannabis industry and the urgent need for sustainable solutions. For Indigenous communities, the path forward lies in prioritizing economic sovereignty through collaborative development of the cannabis and hemp industries. By pooling resources, expertise, and infrastructure, tribes can create a resilient economic network that benefits all members.
At the federal level, comprehensive cannabis reform is essential to unlock the full potential of the industry and address the challenges posed by federal prohibition. This reform should include:
-
Establishing a Federal Regulatory Framework: Congress should establish a comprehensive regulatory framework for cannabis production, testing, labeling, and marketing, ensuring consumer safety and creating a level playing field for businesses.
-
Reforming Section 280E: Congress should reform Section 280E of the Internal Revenue Code to allow cannabis businesses to deduct normal business expenses, reducing their tax burden and promoting economic growth.
-
Promoting Social Equity: Federal cannabis reform should include provisions to promote social equity, ensuring that communities disproportionately affected by the war on drugs have the opportunity to participate in the legal cannabis industry.
The cannabis industry holds immense potential for economic growth, job creation, and social progress. By embracing sensible federal reforms and empowering Indigenous communities to build self-sustaining economies, the United States can unlock this potential and create a more equitable and prosperous future. The federal funding freeze may have been a temporary setback, but it has also served as a catalyst for change, highlighting the urgent need for action and inspiring a renewed commitment to building a more sustainable and equitable cannabis industry for all.
NATIVE AMERICAN CANANBIS IDEAS, READ ON…
DISPENSARIES ON TRIBAL LANDS? ALL LEGAL? HERE IS THE UPDATE!

What Does the Future Look Like in an AI Dominated World?

Cannabis testing lab fraud can be curbed if industry is ‘part of the solution’

The Oregonian: Naked Oregon man wounded by exploding weed pen, lawsuit claims

The Best Thoughtful And Inexpensive Valentine’s Day Gifts

5 Ideas To Have A Fun Single’s Valentine’s Day

Snoop, Wiz, Lil Baby’s latest: Top celeb weed for 2025

Hockey Players Ditching Post Game Beers for Cannabis Edibles and Playstations

The Best Marijuana Strains For Valentine’s Day

Ventura Hall of Flowers is March 19–20—is your business ready?

How Does DOGE and the Federal Funding Freeze Impact the Cannabis Industry?

Distressed Cannabis Business Takeaways – Canna Law Blog™

United States: Alex Malyshev And Melinda Fellner Discuss The Intersection Of Tax And Cannabis In New Video Series – Part VI: Licensing (Video)

What you Need to Know

Drug Testing for Marijuana – The Joint Blog

NCIA Write About Their Equity Scholarship Program

It has been a wild news week – here’s how CBD and weed can help you relax

Cannabis, alcohol firm SNDL loses CA$372.4 million in 2022

A new April 20 cannabis contest includes a $40,000 purse

Your Go-To Source for Cannabis Logos and Designs

UArizona launches online cannabis compliance online course
Trending
-

 Cannabis News2 years ago
Cannabis News2 years agoDistressed Cannabis Business Takeaways – Canna Law Blog™
-

 One-Hit Wonders2 years ago
One-Hit Wonders2 years agoUnited States: Alex Malyshev And Melinda Fellner Discuss The Intersection Of Tax And Cannabis In New Video Series – Part VI: Licensing (Video)
-

 Cannabis 1012 years ago
Cannabis 1012 years agoWhat you Need to Know
-

 drug testing1 year ago
drug testing1 year agoDrug Testing for Marijuana – The Joint Blog
-

 Education2 years ago
Education2 years agoNCIA Write About Their Equity Scholarship Program
-

 Cannabis2 years ago
Cannabis2 years agoIt has been a wild news week – here’s how CBD and weed can help you relax
-

 Marijuana Business Daily2 years ago
Marijuana Business Daily2 years agoCannabis, alcohol firm SNDL loses CA$372.4 million in 2022
-

 California2 years ago
California2 years agoA new April 20 cannabis contest includes a $40,000 purse







