ABC news has this detailed report giving some background on who, how, why & when…
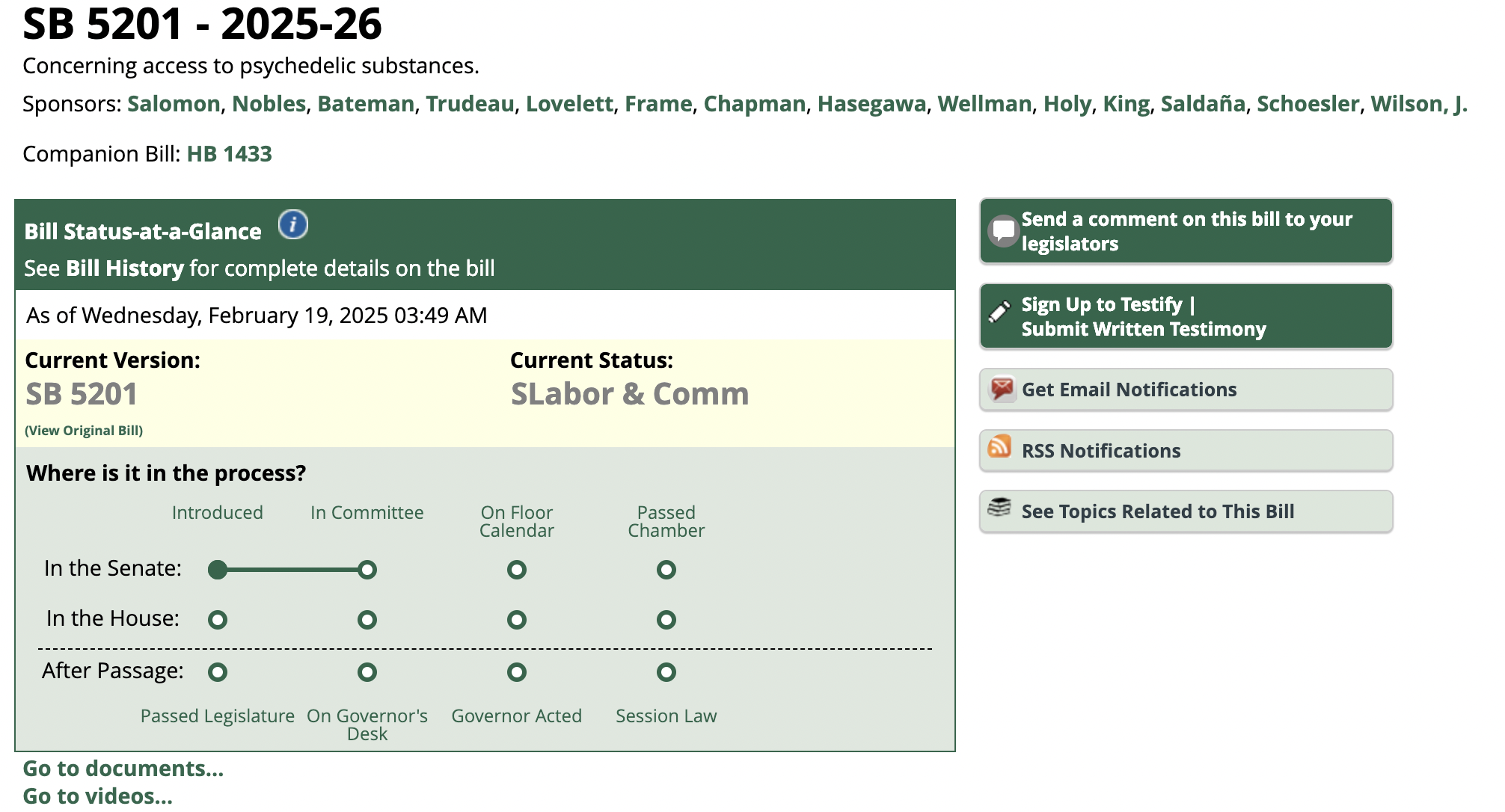
Just three months out from the legalisation of psychedelic medicines for mental health treatment, questions remain among experts about exactly how the drugs will be administered.
In February the Therapeutic Goods Administration (TGA) announced that MDMA and psilocybin — the active ingredient in magic mushrooms — would be reclassified in July to allow them to be prescribed by psychiatrists to treat patients with post-traumatic stress disorder and treatment-resistant depression.
The decision makes Australia the first country in the world to officially recognise the psychedelic substances as medicines.
So what do we know about the drugs and how the scheme will work?
How do psychedelic medicines work?
While trials are still being completed in Australia, researchers in the US and Europe have been exploring the potentials of psychedelic medicine for years.
Professor David Nutt is a neuropsychopharmacologist who is a leading expert in the field, and the head of the psychedelic research group at Imperial College in London.
He told the ABC News Daily podcast the real benefits of drugs like psilocybin and MDMA lie in their ability to break people out of unhelpful cycles which are often a part of mental health conditions.
“Depressed people get locked into thought loops which are repetitive and negative,” he explains.
“They often know they’re not to blame, but they can’t stop thinking that they are to blame. Psychedelics flip you out of that, and they allow you to think differently.”
Professor Nutt uses the analogy of defragmenting a computer hard drive to allow programs to run more fluently.
“We’ve done brain-imaging studies which show that essentially they make the brain more flexible after the trip. People have a more flexible brain.”
What does psychedelic treatment look like?
While treatment modalities are still being researched, the most common approach involves patients having one or two supervised “trips” which typically last for around four to five hours.
After instructions about what to expect, Professor Nutt says the experience typically involves the patient lying down under the supervision of one or two therapists.
“The person listens to music through headphones and normally has eye shades on so they can go into their inner thoughts,” he says.
The following day the patient returns to have what Professor Nutt calls an “integration session” with their therapist where they make sense of and build on the insights gained during the trip.
Professor Nutt says the treatments are so powerful that one or two sessions can be all people need.
“It is truly remarkable,” he says.
“Most people come out with much less depression than they went in.”
“Even in the people with severe depression who failed on ten different medicines, you can still get substantive improvements for weeks or months.”
Professor Nutt believes the move to legalise the drugs for prescription use puts Australia at the cutting edge of what is potentially a revolutionary alternative to traditional mental health treatments.
“I think psychedelics are the biggest advance in psychiatric treatment for 50 years,” he said.
“They’re going to offer options, therapeutic options, for a large proportion of people who don’t get well under current medication.”
Are there any risks in taking psychedelic medicine?
While trials so far have largely shown the medicines to be safe, Professor Nutt cautions that they should only be taken after a detailed medical assessment.
“We don’t give it to people who’ve got either psychotic behaviours or have got first-degree relatives with psychosis,” he explains.
“These drugs don’t cause psychosis, but if you’ve got a vulnerability to psychosis, then they might exacerbate it or bring it on.”
“We exclude those people from our trials and we will continue to exclude them from these therapies.”
At present there are no products containing psilocybin or MDMA included in the Australian Register of Therapeutic Goods that have been evaluated as safe by the TGA.
While the drug regulator notes that adverse effects from the substances are possible, it concludes that these are low risk and are outweighed by the potential benefits they offer.
But some researchers say the data is still insufficient to make confident conclusions about the safety of the medicines.
Professor Susan Rossell is a cognitive neuropsychologist at Swinburne’s Centre for Mental Health which is conducting Australia’s largest research trial into the use of psilocybin for treatment-resistant depression.
“Everything in terms of initial data looks promising, however there’s still so much that we need to know,” she said.
While she believes the drugs have the potential to be ‘a real game changer’, Professor Rossell says she was shocked by the TGAs decision to approve the drugs for medical use while trials were still ongoing.
“Some of the big things we don’t know from the trials is what happens to people immediately after the intervention,” she says.
“Some people have done well, but some people didn’t do well at all and there’s no follow up data.
“We need protocols in place to talk about who is and isn’t suitable for this kind of treatment.”
Professor Rossell believes the TGA’s decision is a “welcome conversation”, however, she says the way the scheme is currently being managed will open people up to risks.
“Unless between now and the first of July there’s a whole pile of things that happen to clarify how this is going to be done. I think people will be harmed,” she said.
But other drug experts say the lack of specific protocols shouldn’t be seen as a reason to prevent the drugs being prescribed to patients who might benefit from them.
Associate Professor David Caldicott is an emergency department doctor who has advocated for the drugs be used for returned soldiers suffering from psychological trauma.
“Should we have had more of the infrastructure developed in anticipation of the legislation or will the legislation prompt the development of the infrastructure?,” he asks.
“My thinking is that the latter is more likely.”
Dr Caldicott draws a parallel to the decision seven years ago to approve cannabis for medicinal purposes.
“Many people were certain there would be medical cannabis psychosis on every street corner,” he said.
“That obviously didn’t eventuate and there are now thousands of Australians who are benefiting from that.”
Who can access the treatment?
In addition to being screened for risk of psychosis, patients wishing to access the drugs also have to have shown themselves to be unresponsive to other available treatments for PTSD or depression.
Professor Nutt believes these criteria provide an appropriate level of protection against harm.
“You’ve got to have failed on two other treatments and you’ve got to have a doctor that’s registered to prescribe it,” he says.
“In those circumstances, I think the risks are very low and the benefits for people who’ve got high suicide risks are really quite substantial.”
Once the laws change in July only registered psychiatrists will be able to prescribe the medicines.
To do so they will need to be approved by an ethics committee registered with the National Health and Medical Research Council and have their authorisation to prescribe granted by a senior medical officer at the TGA.
But Professor Rossell believes questions remain about how psychiatrists will receive training to distribute the medicine and whether they’ll be covered by insurance if they do.
“It’s extraordinarily different from what psychiatrists normally do,” she says.
“I know of 20 or so psychiatrists in Australia who’ve gone overseas to do training on this because there’s no Royal College of Psychiatrists training for this in Australia.”
The lack of accredited training she believes could leave psychiatrists in a grey area in regards to their professional insurance.
“The Australian Health Practitioner Regulation Agency has not endorsed what an accredited prescriber is,” she says.
“How are AHPRA going to decide who gets indemnity insurance because there is no training for those wanting to prescribe these drugs?”
Associate Professor David Caldicott believes the chances of the drugs being administered in what he calls a “rogue fashion” in Australia are very unlikely.
But he says psychiatrists will need to be careful when prescribing the treatment to make sure they are acting within the guidelines.
“You would really have to go quite off-piste to prescribe this, probably to the point that you could threaten your medical registration,” he said.
The TGA says guidance has been provided to psychiatrists wishing to prescribe the drugs and further advice will be released in the coming weeks.
So far Australia is the only country to approve the drugs for prescription use, although Switzerland and the US state of Oregon do permit some psychedelic drugs to be used in a limited way for medical purposes.
Switzerland allows a limited number of psychiatrists to use LSD and MDMA to assist psychotherapy.
Source: https://www.abc.net.au/news/2023-03-03/explainer-australia-mdma-and-psilocybin-for-medicinal-use/102043824


 Cannabis News2 years ago
Cannabis News2 years ago
 One-Hit Wonders2 years ago
One-Hit Wonders2 years ago
 Cannabis 1012 years ago
Cannabis 1012 years ago
 drug testing1 year ago
drug testing1 year ago
 Education2 years ago
Education2 years ago
 Cannabis2 years ago
Cannabis2 years ago
 Marijuana Business Daily2 years ago
Marijuana Business Daily2 years ago
 California2 years ago
California2 years ago