Psychedelics
Australia: Australia To Legalize The Therapeutic Use Of Psilocybine And MDMA

Psychedelics
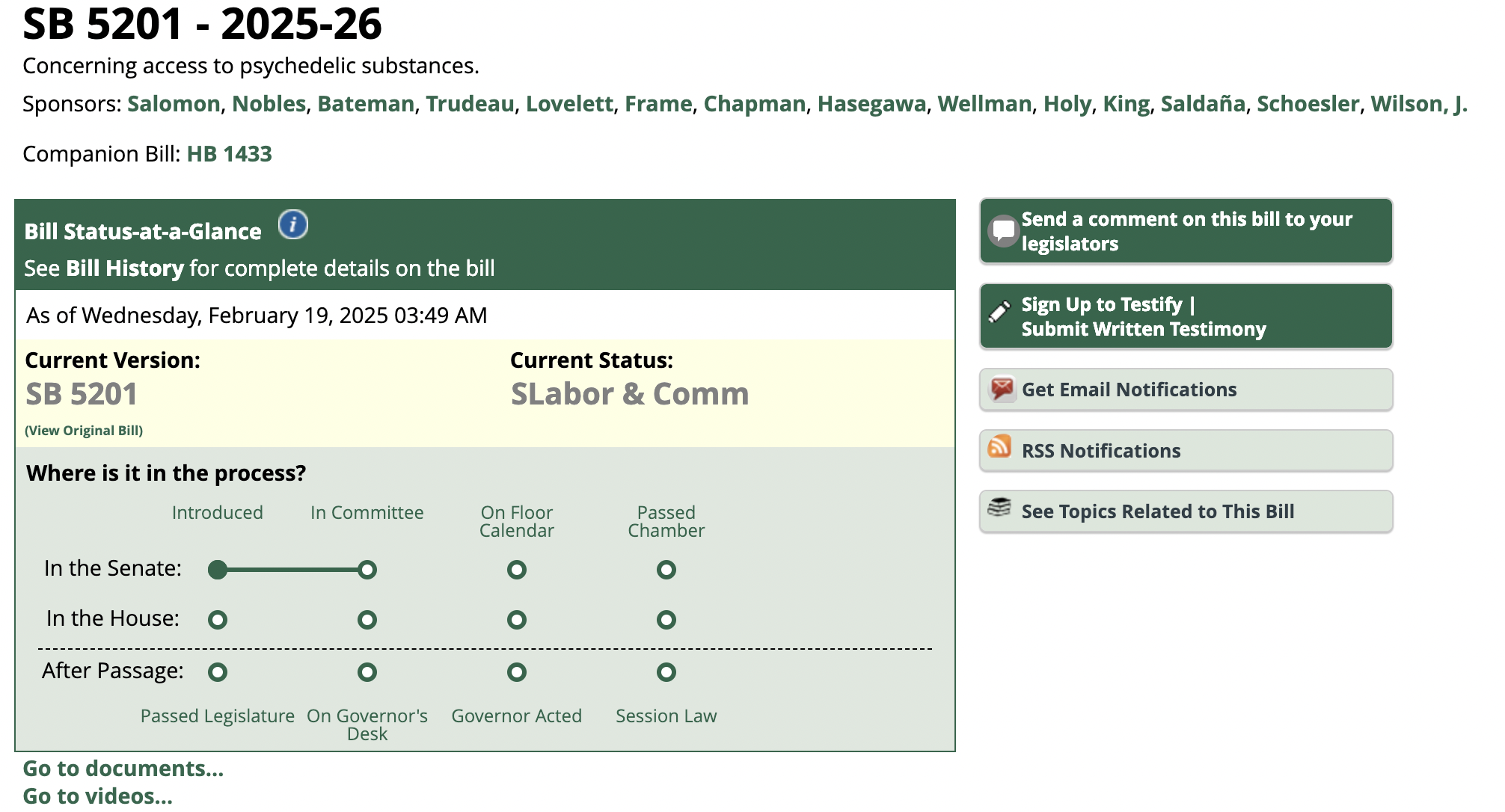
Linked In Post – Jon Dennis, Psychedelic Lawyer: Washington SB 5201, the regulated psilocybin access bill, was considered today by the Senate Committee on Labor & Commerce ( 18 Feb 2025)
Psychedelics
Alert: We are just over a week away from the Natural Medicine Division opening our application process to individuals who are interested in becoming business Owners or Natural Medicine Handlers, and to business applications for Healing Centers, Cultivations, Testing Facilities and Product Manufacturers.
Psychedelics
Mexican “Shamen” on The Run After Actress Dies In Frog Ceremony
-

 Cannabis News2 years ago
Cannabis News2 years agoDistressed Cannabis Business Takeaways – Canna Law Blog™
-
 One-Hit Wonders2 years ago
One-Hit Wonders2 years agoUnited States: Alex Malyshev And Melinda Fellner Discuss The Intersection Of Tax And Cannabis In New Video Series – Part VI: Licensing (Video)
-

 Cannabis 1012 years ago
Cannabis 1012 years agoWhat you Need to Know
-

 drug testing1 year ago
drug testing1 year agoDrug Testing for Marijuana – The Joint Blog
-

 Education2 years ago
Education2 years agoNCIA Write About Their Equity Scholarship Program
-

 Cannabis2 years ago
Cannabis2 years agoIt has been a wild news week – here’s how CBD and weed can help you relax
-

 Marijuana Business Daily2 years ago
Marijuana Business Daily2 years agoCannabis, alcohol firm SNDL loses CA$372.4 million in 2022
-

 California2 years ago
California2 years agoA new April 20 cannabis contest includes a $40,000 purse