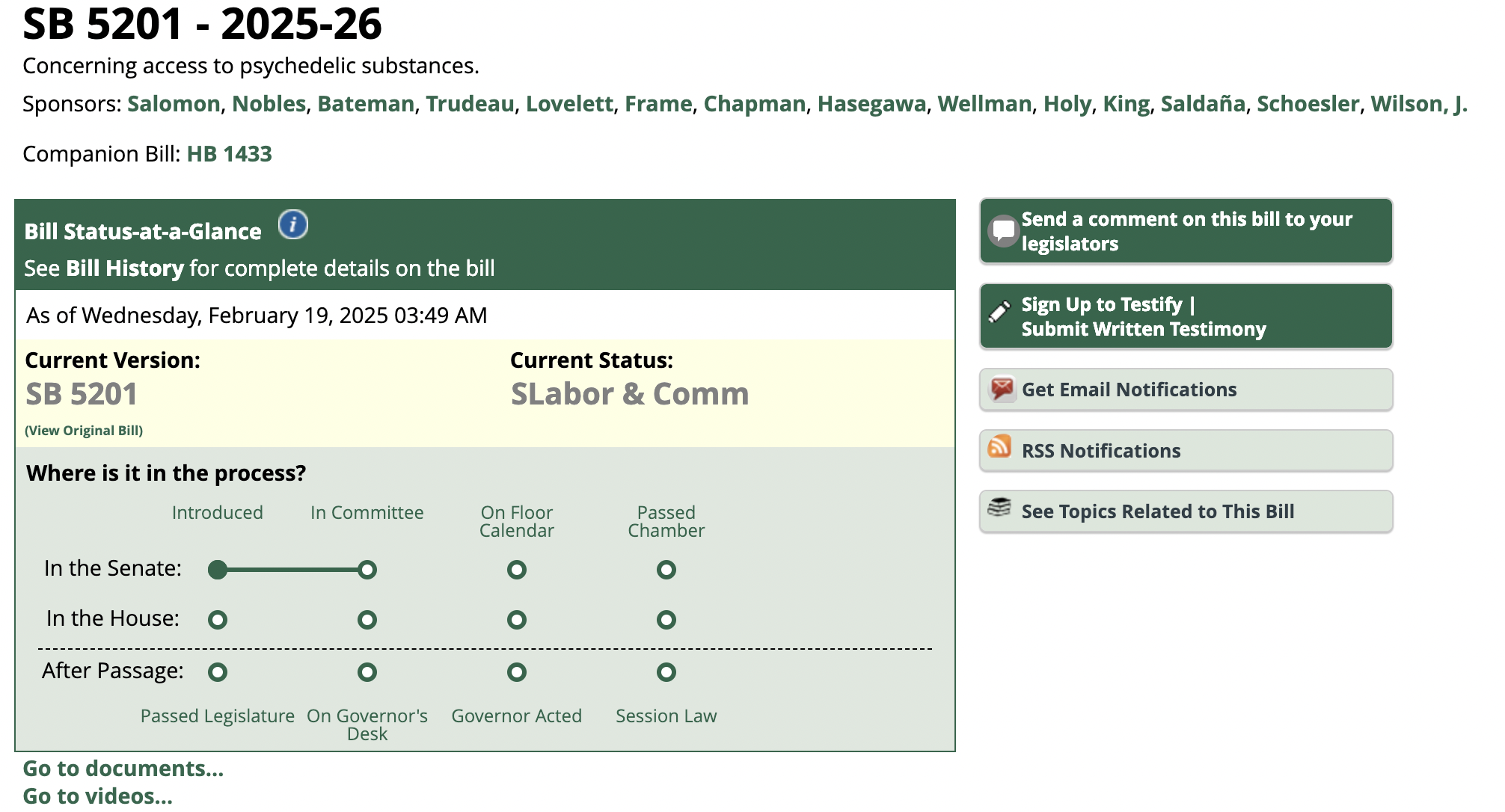
LAS VEGAS, NV, Jan. 31, 2023 (GLOBE NEWSWIRE) — PSYC Corporation (OTC Pink Market: PSYC) (“PSYC”, “PSYC Corp” or the “Company”), parent company to Spotlight Media Corporation (“SMC” or the “wholly owned subsidiary”), a multimedia leader positioned at the intersection where all things psychedelics and cannabis converge, is pleased to provide its shareholders with an overview of recent key corporate updates along with insight into some of the prime objectives the Company is outlining for the year ahead.
Sacha G. Hebbert Joins the PSYC Board
As of this month, the Company is proud to have appointed Sacha G. Hebbert to its Board of Directors alongside PSYC CEO, David Flores and PSYC COO, Michael Berger. In addition to her appointment on the Company’s Board, Ms. Hebbert has also been appointed to the position of Chief Brand Officer (“CBO”) for PSYC Corp and will continue to maintain her position as Chief Operating Officer of the Company’s wholly owned subsidiary, Spotlight Media Corporation (“SMC”).
In her added role as CBO of the parent company, Ms. Hebbert will work in close collaboration with Mr. Flores and Mr. Berger with the ongoing development of PSYC’s brand and image as an evolving publicly traded multimedia leader for the emerging psychedelics sector, and with the objective of helping to identify opportunities to strengthen shareholder value.
Additionally, Ms. Hebbert will continue to play a pivotal leadership role with developing and implementing critical strategies designed to maximize the value of the Company’s current psychedelic-focused media assets operated under SMC such as Psychedelic Spotlight and Bonfire.
“My goal is to ensure that Psychedelic Spotlight continues to grow in domain authority. We’re currently ranked high amongst the top 5 psychedelic media outlets. By the end of this year, I see us in the number one position. Those investing in PSYC will be happy to know that Psychedelic Spotlight is well-positioned as a culture shaper and media partner to the entire B2C market. We continue to tap into the mainstreaming of psychedelics and attract a substantial and curious audience to our platform through provocative and informative content. We’re also building traffic toward Bonfire, our burgeoning community of psychedelic facilitators and seekers. These two platforms, together, create the perfect funnel for service and product providers and consumers in this growing market. By the end of next quarter, we should see a lot of action on our Bonfire platform as we align with strategic partnerships that expand concrete revenue generators.”
Integration of Newly Acquired Media Assets
In September of last year, the Company acquired Technical 420, On The Bids, and Mushroom Stocks from Technical420, LLC. In the months following the acquisition of these media assets, the Company dedicated considerable time to successfully integrating them into SMC as key revenue drivers for the Company.
These efforts, spearheaded by PSYC Chief Operating Officer and SMC Chief Revenue Officer, Michael Berger, have resulted in a steady increase in revenue generation for the Company, which it claims to be reflected as such in it’s soon-to-be-published 2022 annual disclosure and financials. During Q4 of 2022, the Company experienced its strongest revenue performance throughout all of 2022, with approximately $60K generated in gross revenue.
Plans through the first two quarters of 2023 are to continue to implement strategies that are primarily focused on expanding revenue generation capabilities of both Technical 420 and On The Bids. The Company contends that in the months following the acquisition, each of these platforms have emerged as primary revenue drivers for the Company by way of valuable investor and public relations-focused services provided to various publicly traded companies operating in thriving industries such as cannabis and mining and precious metals and where many companies are actively seeking the opportunities to enhance their exposure across the public markets – a key value point offered by Technical 420 and On The Bids.
Furthermore, in the months ahead, the Company plans to apply many of the elements that have have driven the success, from a revenue generation and client satisfaction standpoint, for both Technical 420 and On The Bids over into Mushroom Stocks, and particularly as public companies operating in the psychedelics sector are expected to begin to ramp investor awareness efforts back up throughout 2023.
PSYC COO Michael Berger said, “During the last quarter, our primary objectives have been centered around integrating the business units, finding synergies, and becoming cash flow positive. Since the completion of the acquisition, our websites have generated more than $100,000 of revenue and have secured approximately $75,000 in equity-based agreements. Although market conditions are challenging, we have been able to penetrate new verticals in a cost-efficient manner. We remain bullish on the cannabis and psychedelic industries and believe we have the assets and resources to achieve our primary objectives. Over the next year, we plan to establish Spotlight Media Corp as a media leader in several burgeoning industries and I am confident in our ability to do so.”
Updates on Bonfire and Psychedelic Finder
Following more than 5 months of market testing on the current Bonfire platform, the Company, along with its partner in the project, Digital Acorn, Ltd., has opted to revisit the core fundamentals associated with the platform and with the intent of identifying and developing a more cost effective operations model for the platform, in addition to a more robust and refined monetization strategy that will be more effectively suited for scalability.
The pause on the current iteration of the platform coincides with the resignation of Jack Bunce this month who, over the last several months, had overseen the operations of the Bonfire platform.
In the coming weeks, the Company will work in collaboration with Digital Acorn to identify and select a new leadership team that will focus on successfully ushering Bonfire into the next phase of redevelopment.
The Company contends, however, that it remains cautiously optimistic as to the future potential of Bonfire citing valuable data and information it was successful in gathering throughout the market test phase that, according to the Company, reflects significant market interest in a platform designed with the intent of building a community centered around psychedelic integration.
With regard to Psychedelic Finder, the Company, along with its partners in the project, Nucleus Holding Inc., remain focused and committed to building out this platform which will be an online resource assisting those looking for treatment and care providers.
The companies, however, have agreed to push the official launch of the platform back into later in 2023 as they collectively focus on raising additional capital that will be required to bring some of the intricate details proposed for the platform to fruition.
The companies are intent on bringing to market a platform that goes far beyond what other, similar platforms, operating in this space currently offer.
The Company plans to continue to provide updates on the progress of both Bonfire and Psychedelic Finder in the weeks and months ahead.
Psychedelic Spotlight Continues to Thrive Under a Leaner, More Efficient Business Model
Coming into 2023, PSYC Management made the collective decision to implement various strategic updates to the operational structure of Psychedelic Spotlight. At the core of these updates, was developing a cost-effective plan that will continue to promote the growth of the platform, but under a leaner, and more efficient business model.
Over the course of 2022, PSYC was successful in establishing Psychedelic Spotlight as a top 5 media platform focused exclusively on psychedelics. According to the Company, this top 5 ranking is based on website performance data and analytics gathered from Google such as page views, site visits, and engagement, amongst other metrics.
The Company contends that these metrics play a vital role, as is typically the case with most media platforms, in the ability to monetize the platform through advertisement, sponsorship, and partnership opportunities, and views their current ranking as a competitive advantage in a marketplace that is becoming increasingly competitive.
Since implementing many of the aforementioned updates to the Psychedelic Spotlight business model at the start of the new year, which includes operating the platform with a leaner budget, the Company claims that the metrics tied to its website performance have not dropped off and in fact, the platform continues to hold steady amongst its top competitors and is even demonstrating growth in critical areas such as organic search. This, according to the Company, can be viewed as a demonstration of the success in some of the strategic and hyper-focused growth initiatives Psychedelic Spotlight Director of Marketing, Maria Holyanova, has recently implemented.
The Company plans to continue prioritizing the growth of Psychedelic Spotlight throughout 2023, and while it cannot offer any guarantees, the Company remains committed and highly optimistic in its objective of establishing it as the number one media platform for the emerging sector of psychedelics in the most reasonable time frame possible.
Closing Comments from PSYC CEO, David Flores
“In 2022, we witnessed the culmination of so much of the hard work we had been focused on over the previous two years in terms of developing PSYC into a formidable multimedia leader for psychedelics and other forward-thinking industries such as cannabis. We managed to grow Psychedelic Spotlight into a top 5 media platform for the growing sector of psychedelics, and we brought in new media assets that in addition to providing us with very valuable elements of diversification, have also created a steady, and much-needed revenue stream for us.
The objective here in 2023 is to take what we successfully accomplished last year and expand on it. Cracking the top 5 as a psychedelic’s media platform is a wonderful accomplishment, but make no mistake about it, we aspire to be number one. Bringing in assets that are revenue generating is, in my opinion, a huge step forward for us, but some revenue doesn’t equate to a viable path to profitability, so we’re laser focused on generating even more revenue.
From my perspective, all of these are very important fundamentals that we are meticulously moving into place as part of what we view as a much bigger objective for PSYC. They’re also lending to our ability to have real and very serious conversations related to potential value-driven partnership opportunities and even capital investment opportunities that we simply couldn’t have in years past.
Acquiring what is still, in my opinion, the best ticker symbol for a publicly traded company operating in the psychedelics sector (PSYC), was done so with the intention of developing PSYC into something much more than just another multimedia company operating in the psychedelics space. It was done with the intention of building PSYC into the multimedia company for psychedelics. And as we approach the 3-year mark of the commencement of this journey, this intention, from my perspective, and based on the fundamentals we have put into place, remains as attainable as ever.
There are still many challenges that lie ahead for us in order to earn the trust and credibility within the public markets that we believe is necessary to drive and deliver maximum shareholder value. But what I can assure you is that I, along with the newly formed board of directors and the incredibly talented and dedicated management team we have assembled for the Company, are working tirelessly each and every day to develop solutions that are aimed specifically at overcoming these challenges in the most reasonable manner and time frame possible.
Thank you to all the PSYC shareholders who continue to stand alongside us on this journey. We see you. We hear you, and we are committed to doing everything we can to return the value back to you that we believe you deserve.
About PSYC Corporation (OTC Pink Market: PSYC)
At PSYC Corporation we are integrating media, creativity, and technology to develop and deploy thought-provoking ideas and solutions that are fostering and transforming the approach to some of society’s most pressing matters.
PSYC has expressed its intent and commitment to positioning itself at the forefront of the psychedelic revolution and as a resource center for discovering and understanding the latest research and business opportunities surrounding psychedelic inspired medicines. In conjunction with the FDA’s more open-minded approach to psychedelic medicines, and as several major U.S. cities continue to approve the decriminalization of psilocybin, we believe investors are speculating that the psychedelic boom could be bigger than that of cannabis. PSYC is your source for current investment related news specific to psychedelic medicines and cutting-edge research improving overall health, moving this sector into the mainstream.
We are dedicated to a forward-thinking approach that embraces groundbreaking new technology and innovations and through the vision of business development we intend to continue to evolve into these unchartered territories as the industry leaders of the future.
About Spotlight Media Corporation
Spotlight Media Corporation (“SMC”) (www.spotlightmediacorp.com) is a Nevada Corporation and is a privately held wholly owned subsidiary of PSYC that was incorporated on February 8, 2022. At present time, SMC operates as a multimedia service company for the medicinal psychedelic industry through Psychedelic Spotlight in addition to the developing community-based platform, Bonfire (f/k/a “PsycheDev”). However, management intends, but cannot guarantee the success or profitability, that the business plan for SMC is to potentially expand beyond the medicinal psychedelic industry by way of other multimedia-related opportunities within other niche-style industries like cannabis, health and wellness, and sports such that SMC can make use of the audience it is establishing, across its platforms for cross-promotional opportunities and with the intent of developing a network of interconnected media-focused platforms.
Forward-Looking Statements Disclaimer:
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. In some cases, you can identify forward-looking statements by the following words: “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. Forward-looking statements are not a guarantee of future performance or results and will not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. Forward-looking statements are based on information available at the time the statements are made and involve known and unknown risks, uncertainty and other factors, including the effect of COVID-19 and the success of the current vaccine distribution, the adverse effect of the Omicron variant, that may cause our results, levels of activity, performance or achievements to be materially different from the information expressed or implied by the forward-looking statements in this press release. This press release should be considered in light of all filings of the Company that are disclosed on the OTC Markets.com website and is not incorporated by reference into such reports.
Disclaimer: PSYC Corporation does not in any way encourage or condone the use, purchase, sale or transfer of any illegal substances, nor do we encourage or condone partaking in any unlawful activities. We support a harm reduction approach for the purpose of education and promoting individual and public safety. If you are choosing to use psychedelic substances, please do so responsibly.


 Cannabis News2 years ago
Cannabis News2 years ago
 One-Hit Wonders2 years ago
One-Hit Wonders2 years ago
 Cannabis 1012 years ago
Cannabis 1012 years ago
 drug testing1 year ago
drug testing1 year ago
 Education2 years ago
Education2 years ago
 Cannabis2 years ago
Cannabis2 years ago
 Marijuana Business Daily2 years ago
Marijuana Business Daily2 years ago
 California2 years ago
California2 years ago